Cysteine Targeted Covalent Library
Description
ChemDiv’s Cysteine Targeting Covalent Ligand Library contains 41,378 chemically diverse compounds.
In therapeutic development, covalent drugs stand out as a unique and distinct category. Although they build up a substantial portion of the approved pharmaceuticals, a remarkably small number of these drugs have been intentionally designed for covalent engagement with biological targets [1].
The covalent bonding of small molecule inhibitors to their targets can significantly enhance their potency and/or selectivity compared to other types of chemical interactions. This is achieved through engagement of reactive functional groups that are specifically designed to form covalent bonds with specific sites in target protein molecule, typically side chain of amino acids. Those side chains may be a component of catalytic machinery in the target enzyme, or a non-catalytic side chain located next to the binding pocket. Covalent bonds are markedly stronger than non-covalent interactions, therefore development of the covalent inhibitors paths a way for more efficacious therapeutics then non-covalent drug candidates. As covalent binding is often irreversible, covalent targeted inhibitors exerts extended length of activity to the reversible antagonists, as the target remains inhibited until the protein undergoes degradation and subsequent de-novo synthesis.
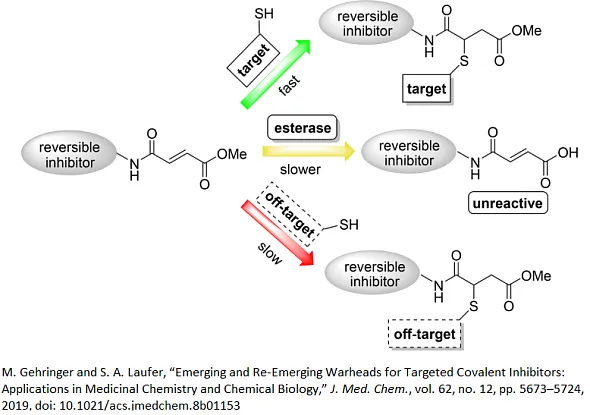
Considerable efforts have been focused recently on targeting noncatalytic cysteine residues, particularly in cysteine proteases and protein kinases, where achieving selectivity to a specific protein family presents a research challenge. The attractiveness of cysteine as a target partly comes from its relatively scarce presence in protein space and high nucleophilicity. The thiolate form of cysteine has demonstrated the ability to form covalent bonds with a broad spectrum of covalent warheads, exhibiting varied reactivity levels. Group of Michael acceptors is prominent example, showcasing the versatile reactivity and potential of cysteine in covalent bonding interactions [2].
Publications
[1] S. De Cesco, J. Kurian, C. Dufresne, A. K. Mittermaier, and N. Moitessier, “Covalent inhibitors design and discovery,” Eur. J. Med. Chem., vol. 138, pp. 96–114, 2017, doi: 10.1016/j.ejmech.2017.06.019.[2] R. Lonsdale and R. A. Ward, “Structure-based design of targeted covalent inhibitors,” Chem. Soc. Rev., vol. 47, no. 11, pp. 3816–3830, 2018, doi: 10.1039/c7cs00220c.
[3] M. Gehringer and S. A. Laufer, “Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology,” J. Med. Chem., vol. 62, no. 12, pp. 5673–5724, 2019, doi: 10.1021/acs.jmedchem.8b01153.
[4] P. A. Jackson, J. C. Widen, D. A. Harki, and K. M. Brummond, “Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions,” J. Med. Chem., vol. 60, no. 3, pp. 839–885, 2017, doi: 10.1021/acs.jmedchem.6b00788.
[5] T. Barf and A. Kaptein, “Irreversible protein kinase inhibitors: Balancing the benefits and risks,” J. Med. Chem., vol. 55, no. 14, pp. 6243–6262, 2012, doi: 10.1021/jm3003203.
[6] R. A. Bauer, “Covalent inhibitors in drug discovery: From accidental discoveries to avoided liabilities and designed therapies,” Drug Discov. Today, vol. 20, no. 9, pp. 1061–1073, 2015, doi: 10.1016/j.drudis.2015.05.005.