Human Transcription Factors Annotated Library
Description
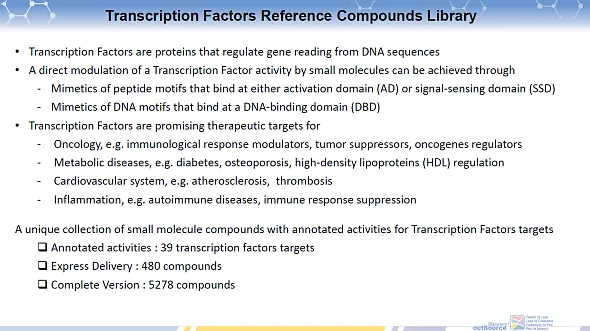
•Transcription Factors are proteins that regulate gene reading from DNA sequences
•A direct modulation of a Transcription Factor activity by small molecules can be achieved through
-Mimetics of peptide motifs that bind at either activation domain (AD) or signal-sensing domain (SSD)
-Mimetics of DNA motifs that bind at a DNA-binding domain (DBD)
•Transcription Factors are promising therapeutic targets for
-Oncology, e.g. immunological response modulators, tumor suppressors, oncogenes regulators
-Metabolic diseases, e.g. diabetes, osteoporosis, high-density lipoproteins (HDL) regulation
-Cardiovascular system, e.g. atherosclerosis, thrombosis
-Inflammation, e.g. autoimmune diseases, immune response suppression
A unique collection of small molecule compounds with annotated activities for Transcription Factors targets
- Annotated activities : 36 transcription factors targets
- Express Delivery : 480 compounds
- Complete Version : 5278 compounds
Library Composition
Data sources of annotations : Pharos, ChEMBL 25, PubChem, PubMed, Current Patent Literature (CAS, Integrity)
IDNUMBER – ChemDiv Catalog ID (in some instances the same IDNUMBER might have multiple annotation entries due to multiple data sources or because having activity against multiple similar targets);
UNIPROT – SwissProt and ChEMBL Target accesion ID;
Type – character of the measured activity;
Value – Active compounds selection criteria, included only compounds with reported activities < 5 µM;
pubmed_id – PubMed record entry;
doi, patent_id – journal or patent reference to a publication of original data;
For screening data extracted from PubChem, see column assay_description for entry names PUBCHEM_BIOASSAY
Example of Annotations - an Excel file structure
|
IDNUMBER |
UNIPROT |
Target Name |
Type |
Relation |
Value |
Units |
pubmed_id |
doi |
patent_id |
Target Description |
assay_description |
|
1431-2228 |
Q9UBN7 |
Histone deacetylase 6 |
IC50 |
= |
1590 |
nM |
|
|
US-8748451-B2 |
Histone deacetylase 6 |
Activity Assay: HDAC assay is performed using fluorescently-labeled acetylated substrate which comprises an acetylated lysine side chain. |
|
0896-4245 |
O60341 |
Lysine-specific histone demethylase 1 |
IC50 |
= |
218 |
nM |
|
|
US-8987335-B2 |
Lysine-specific histone demethylase 1A |
Inhibitor Screening Assay: The primary assay for compound inhibitory activity |
|
1741-0974 |
Q9Y294 |
Histone chaperone ASF1A |
IC50 |
= |
600 |
nM |
25582598 |
10.1016/j.bmcl.2014.11.067 |
|
Histone chaperone ASF1A |
Inhibition of His-tagged human Asf1a binding with H3/H4 by ALPHA assay |
|
G266-0266 |
P55201 |
Peregrin |
IC50 |
= |
100 |
nM |
25408830 |
10.1021/ml5002932 |
|
Peregrin |
Binding affinity to 6H-Flag-tagged Tev-BRPF1 (622-738 aa) (unknown origin) by TR-FRET |
|
8015-7476 |
O96028 |
Histone-lysine N-methyltransferase NSD2 |
AC50 |
= |
113 |
nM |
|
|
|
Histone-lysine N-methyltransferase NSD2 |
PubChem BioAssay. Development of Small Molecule Probes of the Histone Methyltransferase NSD2 |
|
5067-3370 |
O00255 |
Menin/Histone-lysine N-methyltransferase MLL |
Potency |
= |
0.1 |
nM |
|
|
|
Menin |
PUBCHEM_BIOASSAY: qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias |
|
8016-5992 |
Q9BY41 |
Histone deacetylase |
IC50 |
= |
515 |
nM |
11831887 |
10.1021/jm015568c |
|
Histone deacetylase 8 |
Concentration required to inhibit human Histone deacetylase (HDAC) enzyme by 50% |
|
0402-0164 |
Q9BY41 |
Histone deacetylase |
Ki |
= |
8.21 |
nM |
19520580 |
10.1016/j.bmc.2009.05.042 |
|
Histone deacetylase 8 |
Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assay |
|
1822-0862 |
Q9Y6K1 |
DNMT3A2/3L complex |
IC50 |
= |
500 |
nM |
25406944 |
10.1021/jm500843d |
|
DNA (cytosine-5)-methyltransferase 3A |
Inhibition of His6-tagged human recombinant DNMT3A/DNMT3L |
|
R092-0055 |
P04150 |
Glucocorticoid receptor |
Ki |
= |
5.5 |
nM |
8627601 |
10.1021/jm950747d |
|
Glucocorticoid receptor |
Binding affinity was determined for human glucocorticoid receptor(hGR). |
Publications
1.J. Med. Chem. 2016 59(4):1642-1647. Discovery of a Chemical Tool Inhibitor Targeting the Bromodomains of TRIM24 and BRPF. Bennett J Fedorov O Tallant C Monteiro O Meier J Gamble V Savitsky P Nunez-Alonso GA Haendler B Rogers C Brennan PE Müller S Knapp S.
2.J. Med. Chem. 2013 56(4):1772-1776. Potent and selective inhibition of histone deacetylase 6 (HDAC6) does not require a surface-binding motif. Wagner FF Olson DE Gale JP Kaya T Weïwer M Aidoud N Thomas M Davoine EL Lemercier BC Zhang YL Holson EB.
3.J. Med. Chem. 2015 58(6):2569-2583. Targeting DNA methylation with small molecules: what's next? Erdmann A Halby L Fahy J Arimondo PB.
4.J. Med. Chem. 2007 50(26):6519-6534. Nonsteroidal glucocorticoid agonists: tetrahydronaphthalenes with alternative steroidal A-ring mimetics possessing dissociated (transrepression/transactivation) efficacy selectivity. Biggadike K Boudjelal M Clackers M Coe DM Demaine DA Hardy GW Humphreys D Inglis GG Johnston MJ Jones HT House D Loiseau R Needham D Skone PA Uings I Veitch G Weingarten GG McLay IM Macdonald SJ.
5.Proc. Natl. Acad. Sci. U.S.A. 2007 104(49): 19244-19249. Antiinflammatory glucocorticoid receptor ligand with reduced side effects exhibits an altered protein-protein interaction profile. Miner JN Ardecky B Benbatoul K Griffiths K Larson CJ Mais DE Marschke K Rosen J Vajda E Zhi L Negro-Vilar A.
6.ACS Med. Chem. Lett. 2014 5(12):1318-1323. Optimization of drug-like properties of nonsteroidal glucocorticoid mimetics and identification of a clinical candidate. Harcken C Riether D Liu P Razavi H Patel U Lee T Bosanac T Ward Y Ralph M Chen Z Souza D Nelson RM Kukulka A Fadra-Khan TN Zuvela-Jelaska L Patel M Thomson DS Nabozny GH.
7.Bioorg. Med. Chem. Lett. 2013 23(19):5442-5447. Discovery of potent and selective nonsteroidal indazolyl amide glucocorticoid receptor agonists. Sheppeck JE Gilmore JL Xiao HY Dhar TG Nirschl D Doweyko AM Sack JS Corbett MJ Malley MF Gougoutas JZ Mckay L Cunningham MD Habte SF Dodd JH Nadler SG Somerville JE Barrish JC.
8.Bioorg. Med. Chem. Lett. 2014 24(22):5265-5267. Identification of the first inverse agonist of retinoid-related orphan receptor (ROR) with dual selectivity for RORβ and RORγt. Gege C Schlüter T Hoffmann T.
9.Bioorg Med Chem 2017 25(2):471-482. Structure-anticonvulsant activity studies in the group of (E)-N-cinnamoyl aminoalkanols derivatives monosubstituted in phenyl ring with 4-Cl 4-CH3 or 2-CH3. Gunia-Krzyżak A Żelaszczyk D Rapacz A Żesławska E Waszkielewicz AM Pańczyk K Słoczyńska K Pękala E Nitek W Filipek B Marona H.
10.J. Med. Chem. 2011 54(24):8616-8631. Identification of benzoxazin-3-one derivatives as novel potent and selective nonsteroidal mineralocorticoid receptor antagonists. Hasui T Matsunaga N Ora T Ohyabu N Nishigaki N Imura Y Igata Y Matsui H Motoyaji T Tanaka T Habuka N Sogabe S Ono M Siedem CS Tang TP Gauthier C De Meese LA Boyd SA Fukumoto S.