Inhibitors of beta-Catenin Signaling
ChemDiv’s Library of the of beta-Catenin Signaling Pathway Inhibitors contains 8,000 compounds.
β-Catenin is a multifunctional protein involved in crucial pathways in cellular and developmental processes. It is commonly categorized into two primary functions: as an adhesion molecule facilitating cell-cell interactions and as a signaling molecule that transmits extracellular signals to the nucleus, thereby influencing gene expression. β-Catenin interacts with numerous partner proteins, reflecting its role as a central node in various cellular signaling pathways. The Wnt/β-catenin signaling pathway, a subset of those networks, plays a pivotal role in diverse biological systems, including stem cell regulation, embryonic development, and adult organ function. Dysregulation of the Wnt/β-catenin pathway components have been linked to a range of diseases, notably several cancers and degenerative disorders.
ChemDiv has developed a novel library of β-catenin inhibitors/modulators. This collection comprises drug-like compounds specifically designed to modulate the protein-protein interactions (PPIs) between β-catenin and various physiological partner proteins. The library was curated using ChemDiv's in-house structural biology insights, molecular dynamics simulations, virtual screening of unique chemical entities, and medicinal chemistry techniques for the selection and prioritization of potential candidates. It includes compounds targeting 'druggable' sites on β-catenin, identified through their specific topological features that facilitate those interactions.
1. ß-catenin: structure and functions.
β-Catenin is a versatile and evolutionarily conserved protein, essential in metazoans for various developmental and homeostatic processes. Specifically, β-catenin is a key structural component of cadherin-based cell adhesion junctions and acts as a primary nuclear effector in the canonical Wnt signaling pathway.
Belonging to the catenin protein family, β-catenin was first identified in 1989 alongside its counterparts, α and γ-catenin, distinguished by molecular weights of 102, 88, and 80 kDa, respectively. The structure of β-catenin is characterized by an N-terminal domain (NTD), a central core of 12 Armadillo (Arm) repeats (R1–12), and a C-terminal domain (CTD).
In vertebrates, the catenin family, encompassing β-catenins, p120-catenin, and plakophilins 1 and 3, is defined by a large number of Arm repeats. These proteins together form a complex and multifunctional network. Imbalances in the structural integrity and signaling functions of β-catenin can lead to various diseases, prominently including uncontrolled cell growth, cancer, and metastasis.
β-Catenin is involved in key biological processes:
● Cell adhesion mechanisms: It maintains cell-cell adhesion integrity;
● Signal transduction: It plays a pivotal role in transmitting signals within cells;
● Anti-inflammatory effects: β-catenin has been implicated in modulating inflammatory responses.
2. WNT/beta-catenin pathway: components, mechanisms, and functions.
Wnt/β-catenin signaling is a highly conserved evolutionary pathway that regulates critical cellular functions, in particular, proliferation, differentiation, migration, genetic stability, apoptosis, and stem cell renewal. This pathway operates through either the canonical or non-canonical mechanisms, determined by the involvement of β-catenin in signal transduction. β-Catenin is key component of the cadherin protein complex, which stabilization is required for the Wnt/β-catenin signaling activation.
Numerous challenges and pitfalls are observed in therapies targeting Wnt signaling pathways, due to their multifaceted aberrations, underscoring their significance in developmental therapeutics. The role of the Wnt/β-catenin pathway in modulating immune cell infiltration in tumor microenvironments has recently gained attention, particularly for its potential implications in immunotherapy.
The components of the Wnt/β-catenin pathway were initially identified by Nusse and Varmus in 1982 during their study of oncogenic breast tumor viral diseases (MMTV) [1]. They hypothesized that proviral insertion at specific sites was a mechanism of carcinogenesis, leading to the discovery of the INT1 gene linked to this pathway. Concurrent research in developmental biology and genetics in Drosophila revealed that INT1 is homologous to the Drosophila segment polarity gene [2]. Further studies confirmed that human INT1 is markedly similar to mouse INT1, and the highly conserved nature of this pathway was found across different species [3]. Additional research identified several genes, such as INT2, INT3, and INT4, at MMTV provirus insertion sites in tumors, linking them to various developmental pathways [4-6]. For example, INT2 belongs to the fibroblast growth factor (FGF) family, specifically FGF-3, while INT3 is associated with the NOTCH gene family [7, 8]. Due to the confusion arising from the "INT" nomenclature, a consensus led to the adoption of the "WNT" (wingless-type MMTV integration site) designation. INT1, now known as WNT1, became a prototype member [9].
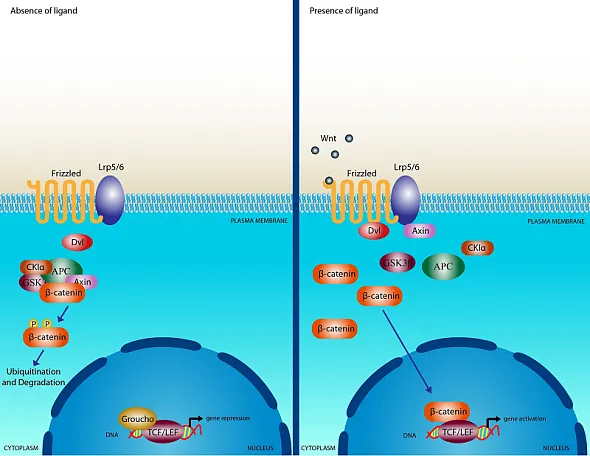
WNTs (translated products of the WNT gene) are cysteine-rich glycoproteins secreted into the extracellular matrix, activating receptor-mediated signaling [10]. The WNT protein family comprises at least 19 members, which are conserved from invertebrates to mammals [11]. WNT proteins bind to the extracellular domain of Frizzled receptors, part of the G protein-coupled receptor superfamily, and disrupt the β-catenin destruction complex (a tertiary complex formed by axin, adenomatous polyposis coli (APC), CK1a and GSK3β), leading to cytoplasmic accumulation of β-catenin.
The T-cell factor/lymphoid enhancer factor-1 (TCF/Lef1) is a transcription complex mediates canonical gene transcription induced by WNT [12, 13]. β-Catenin translocates to the nucleus, where it activates the TCF/Lef1 transcription complex [14-16]. Additionally, β-catenin is involved in cell adhesion, accumulating at intercellular junctions [17, 18]. The canonical Wnt/β-catenin signaling pathway is depicted on slide 1 in the deck above. Beyond the canonical pathway, WNT can also activate β-catenin-independent, non-canonical pathways [19]. The integrated Wnt pathway concept combines canonical and non-canonical pathways, integrating multiple inputs at the receptor level and subsequent intracellular responses [20].
The Wnt/β-catenin pathway is crucial in the pathogenesis of various cancers, and recent insights into its role in immune regulation have ignited renewed interest in this field.
3. Potential Mutation Variants in WNT Pathway Components and Their Impact on Tumorigenesis
Current research data indicate that mutations play a crucial role in the abnormal interaction of β-catenin with its destruction complex, leading to the development of various tumors [4]. Numerous studies have identified β-catenin as a vital modulator of tumor cell proliferation and survival [5]. This protein also supports tumor growth by stimulating angiogenesis through its involvement in regulating the expression of vascular endothelial growth factor [9].
β-Catenin contributes to the mechanisms of tumor metastasis by enhancing cellular migration and invasion capabilities. Specifically, it regulates the expression of matrix metalloproteinase genes, which products are integral to tissue remodeling, angiogenesis, proliferation, cell migration and differentiation, and apoptosis. Furthermore, β-catenin alters the expression of target genes in fibroblasts, macrophages, mesenchymal stem cells, and endothelial cells, thereby influencing the tumor microenvironment. This modulation is directly significant for the growth and progression of malignant tumors. Dysfunctional signaling and adhesive function of β-catenin have been observed in colorectal cancer, hepatocellular carcinoma, prostate cancer, thyroid cancer, and other neoplasms. The involvement of the Wnt/β-catenin pathway in several malignancies highlights its importance in oncological research and most common types of cancer:
- Colorectal cancer (CRC)
The role of the Wnt/β-catenin pathway in carcinogenesis was initially described in relation to the APC gene mutations. Those aberrations, commonly acquired early in the pathogenesis of most colon cancers (accounting for over 80%), lead to the cytosolic accumulation of β-catenin, and in combination with TCF/Lef1, β-catenin is transported into the nucleus. Intranuclearly, it functions as a transcription factor, promoting cell proliferation [21, 22]. Nuclear expression of β-catenin has been linked to more aggressive cancer biology. For instance, a study reported nuclear expression of β-catenin in 18 of 25 (72%) cases of ulcerative colorectal cancer (CRC), compared to only 7 of 26 (26.9%) cases of polypoid CRC. That correlation was found to be independent of the APC mutation and E-cadherin expression [23]. The Wnt/β-catenin pathway is also involved in cross-talk with the Hippo/YAP pathway. Consavage et al. found that β-catenin/TCF4 complexes bind to the DNA enhancer element in the first intron of the YAP gene. This binding stimulates YAP expression in CRC cells, thereby promoting carcinogenesis [24]. Furthermore, the Hippo-YAP signaling pathway may act downstream of APC, independent of its role in the β-catenin degradation complex [25].
The Wnt pathway is implicated in sustaining cancer stem cell (CSC) proliferation in colorectal cancer. In vitro studies suggest that chronic 'stemness' induced by chemotherapeutic stress correlates with attenuated Wnt signaling [26]. Vermeulen et al. observed high activity of the Wnt pathway predominantly in tumor cells located near stromal myofibroblasts. These myofibroblasts are thought to secrete factors like hepatocyte growth factor (HGF), which activate β-catenin-dependent transcription, thereby maintaining CSC clonogenicity and reestablishing the CSC phenotype in more differentiated tumor cells both in vitro and in vivo [27]. Additionally, HGF induces nuclear translocation of β-catenin via Met/β-catenin dissociation, following a Wnt-independent pathway [28].
- Noncolorectal gastrointestinal cancer
Mutations in β-catenin have been identified as key factors in the early stages of carcinogenesis, particularly in activating the Wnt pathway in gastric and non-hepatitis-associated hepatocellular cancers [29, 30]. Cholangiocarcinoma has also been shown to be a Wnt-dependent type of cancer that thrives on canonical activation of the Wnt pathway. Activation of Wnt/β-catenin signaling by pluripotent mesenchymal stem cells recruited to the tumor site appears to play a central role in modulating the tumor microenvironment, promoting metastasis growth, and chemotherapeutic resistance in cholangiocarcinoma [31]. M2-polarized tumor-associated macrophages (TAMs) in the surrounding stroma are known to maintain a substantially upregulated Wnt pathway in cancer cells [32].
- Desmoid tumors
Crago et al. demonstrated that mutations in the APC and CTNNB1 genes (which encodes β-catenin) are prevalent in desmoid tumors, occurring in 111 out of 117 cases (95%). Even tumors with a true wild-type CTNNB1 genotype, as determined by next-generation sequencing, can exhibit genomic alterations that activate the WNT pathway. An example of such alterations is a chromosome 6-loss coupled with a BMI1 mutation. This finding underscores the significance of Wnt/β-catenin activation as a key regulatory pathway in the initiation of desmoid tumors [33].
- Adrenocortical carcinoma
Activation of the Wnt/β-catenin pathway has been identified as an independent predictor of both overall and disease-free survival in patients with surgically removed primary adrenocortical carcinoma. The presence of β-catenin nuclear staining is significantly correlated with higher tumor stages, increased risk scores, frequent necrosis, mitosis, and the presence of other associated mutations [38]. Animal research study results demonstrated that β-catenin can act as an oncogene in the adrenal gland. This role is characterized by the progressive hyperplasia of subcapsular cells and ectopic expansion of spongiocytes and subcapsular cells, leading to dysplasia. Additionally, prolonged activation of β-catenin typical leads to pronounced differentiation defects, manifesting in primary hyperaldosteronism and the development of malignant properties, specifically uncontrolled neovascularization and local invasion. [39].
Melanoma. The role of Wnt/β-catenin activation in the metastatic spread of melanoma is still considered controversial. While β-catenin signaling reduces the migration of melanocytes and melanoma cell lines in vitro, it paradoxically promotes lung metastasis in a melanoma mouse model driven by NRAS mutation. β-Catenin is also a significant factor in the spread of melanoma metastasis to lymph nodes and lungs in a mouse model characterized by melanocyte-specific PTEN loss and BRAF (V600E) mutation. Additionally, the level of β-catenin affects tumor differentiation and regulates both MAPK/Erk and PI3K/Akt signaling pathways. The concurrent activation of Wnt/β-catenin and AKT pathways has been related to chemoresistance and enhanced invasiveness in melanoma cell lines [32].
Glioblastoma multiforme. The Wnt pathway components are commonly overexpressed in glioblastoma multiforme (GBM) tumors. PLAG2 overexpression may play a role in triggering upregulation of Wnt6, FZD9, and FZD2, which ultimately leads to the maintenance of stemness in GBM stem cells. β-catenin also increases the expression of the DNA repair enzyme O6- methylguanine-DNA-methyltransferase (MGMT) via Tcf/Lef binding located in the 5'-flanking regulatory region of hmMGMT. Genetic or pharmacological inhibition of Wnt/β-catenin signaling reduces MGMT expression and enhances the cytotoxic effects of temozolomide [15].
Renal cell cancer (RCC). Elevated levels of β-catenin expression are linked with poor prognosis, advanced staging, nodal involvement, vascular invasion, and sarcomatoid differentiation in rRCC. Comprehensive omics analyses, including both methylome and transcriptome assays, have further emphasized the significant role of the Wnt/β-catenin signaling pathway in RCC pathogenesis. Mutations in such genes as GCN1L1, MED12, and CCNC, which are part of the CDK8 mediator complex that directly regulates β-catenin-driven transcription, were identified in 16% of RCC cases. [16].
Osteosarcoma. Expression of the Wnt LRP-5 receptor correlates with worse event-free survival in patients with osteosarcoma [17]. Targeting LRP5 receptor signaling via a dominant-negative receptor form inhibits tumor growth and metastasis rate and reduces the expression of biomarkers associated with cancer cell invasiveness in animal osteosarcoma models.
5. Regulation of the WNT/beta-catenin pathway: activators and inhibitors.
Several natural agents demonstrate antitumor activity by modulating the canonical Wnt signaling pathway. Curcumin, extracted from the rhizome of Curcuma longa, affects the Wnt signaling pathway and exerts antitumor effects in various cancers, including melanoma, lung, breast, and colon carcinomas, endothelial carcinoma, gastric, and hepatocellular carcinoma. 3,3'-Diindolylmethane (DIM), derived from cruciferous vegetables, inhibits proliferation in colon and colorectal cancer cells via the Wnt/β-catenin pathway, showing potential as a chemopreventive or chemo-radiosensitizer in cancer therapy to prevent tumor recurrence.
Formononetin, isolated from red clover, displays antitumor activity in breast cancer and glioma cells with high IC50 values. Enhancing its potency, formononetin was chemically modified with a coumarin unit to create derivative 10 using a molecular hybridization strategy. This analog demonstrated antiproliferative effects through the Wnt/β-catenin pathway in gastric cancer [3].
Wogonin, a major flavonoid from Scutellaria radix, reduces intracellular Wnt protein levels and promotes β-catenin degradation for proteasomal processing. Gigantol, a bibenzyl compound from orchid species, has been reported to inhibit Wnt/β-catenin signaling by downregulating phosphorylated LRP6 and cytosolic β-catenin in breast cancer cells. Additionally, echinacoside, a phenylethanoid glycoside from Tibetan herbs, significantly reduces tumor growth by modulating Wnt/β-catenin signaling. Nimbolide, a limonoid from neem tree leaves, suppresses canonical Wnt signaling and induces intrinsic apoptosis in hepatocarcinoma cells. Isoquercitrin, a natural flavonol compound, inhibits Wnt/β-catenin signaling, regulating the translocation of β-catenin to the nucleus. Tryptonide, a diterpenoid epoxide from Tripterygium wilfordii, effectively inhibits canonical Wnt/β-catenin signaling by targeting β-catenin’s C-terminal transcription domain or the related nuclear components, inducing apoptosis in Wnt-dependent cancer cells. Furthermore, experimental results indicate that the fungus Exobasidium vexans and its subcomponent atranorin inhibit lung cancer cell motility and tumorigenesis by impacting β-catenin nuclear import and downstream β-catenin/LEF target genes.
5. Wnt/beta-catenin pathway inhibitors in clinical trials
PORCN inhibitors. Porcupine membrane-bound O-acyltransferase (PORCN), belonging to the membrane-bound O-acyltransferase (MBOAT) family, plays a key role in the secretion of Wnt ligands [30]. Several PORCN-targeting inhibitors have been developed to prevent the palmitoylation of Wnt proteins in the endoplasmic reticulum, thereby inhibiting their secretion [13, 24]. This strategy of blocking Wnt acylation using PORCN inhibitors to suppress Wnt secretion was found to be an effective treatment approach. WNT974 (LGK974), an orally administered small-molecule inhibitor, significantly reduces the viability of epithelial ovarian cancer (EOC) cells in vitro and inhibits tumor growth in vivo. In preclinical mouse models of EOC, the combination of WNT974 with paclitaxel demonstrates enhanced antitumor efficacy.
Wnt/FZD antagonists. Antagonizing Wnt ligands and FZD receptors effectively suppresses the canonical Wnt signaling pathway, presenting a promising approach for cancer therapy. Ipafricept (OMP54F28; IPA) is a novel recombinant fusion protein, consisting of the cysteine-rich domain of FZD8 coupled with a human IgG1 Fc fragment [34]. This configuration enables Ipafricept to directly engage with Wnt ligands, competing with the FZD8 receptor for these ligands and thereby inhibiting Wnt-regulated cellular processes. [33].
OMP-18R5 (vantictumab) is a monoclonal antibody that targets FZD1, FZD2, FZD5, FZD7, and FZD8. It inhibits cell proliferation, thereby reducing the growth of breast, pancreatic, colon, lung, and head and neck cancers in mouse models. The efficacy of OMP-18R5 against these types of cancers is currently being evaluated in several Phase I studies.
LRP5/6 inhibitors. LRP5/6 is a key co-receptor of Wnt pathway, and its phosphorylation facilitates activation of the Wnt/β-catenin signaling. The molecular complex composed of Wnt, FZD, LRP5/6, and DVL forms a structural domain for interaction with axin, which disrupts β-catenin degradation. BMD4503-2, a quinoxaline derivative, has been identified as a novel small-molecule inhibitor that blocks interactions between LRP5/6 and sclerostin, as confirmed through pharmacophore-based virtual screening and a few in vitro assays. By competitively binding to the LRP5/6-sclerostin complex, BMD4503-2 has the potential to restore the diminished activity of the Wnt/β-catenin signaling pathway.
DVL inhibitors. DVL plays a crucial role in Wnt signaling by recruiting components of the β-catenin destruction complex to the cell membrane. It binds to the cytoplasmic carboxyl terminus of FZD proteins through its specific domain. Agents such as NSC668036, FJ9, and 3289-8625 have been found to prevent the DVL-PDZ interaction, consequently inhibiting the signal transduction pathway.
6. Clinical study results confirming efficacy and safety of ß-catenin inhibitors against cancers
There are several therapeutic strategies focused on inhibiting the Wnt pathway that have been developed so far, resulting in numerous drug compounds reaching early clinical trial phases. PORCN has become a promising molecular target for the treatment of cancers induced by Wnt aberrations [3]. The addition of palmitoyl groups to Wnt proteins is catalyzed by PORCN, a biochemical process known as palmitoylation or S-acylation in the endoplasmic reticulum that enhances the secretion of Wnt into the cytoplasm, thus promoting tumor growth. WNT974, a PORCN inhibitor, had a cytostatic effect on ovarian cancer cells in vitro and reduced tumor growth and metastasis rate in animal models of the head and neck squamous cell carcinoma [19].
A phase I/II study is ongoing evaluating WNT974 in combination with LGX818, a specific BRAF inhibitor, and cetuximab in patients with metastatic colorectal cancer carrying both WNT and BRAF mutations (NCT02278133). Another study is in active phase evaluating WNT974 in patients with metastatic head and neck squamous cell carcinoma (NCT02649530).
WNT agonists, in particular the ones inhibiting WNT-5a activation, have been shown to reduce metastasis rate. Upregulated WNT-5a signaling suppresses endothelial tumor cell migration and invasion and inhibits metastasis in breast cancer model in vivo. Foxy-5 is a WNT-5a mimic hexapeptide that binds and activates the WNT-5a, Frizzled-2, and Frizzled-5 receptors [6]. Two Phase I clinical trials are in progress to evaluate the efficacy of Foxy-5 in developed solid tumors (breast, colon, and prostate) knocked-out or downregulated WNT-5a protein expression (NCT02020291 and NCT02655952).
CWP232291 is a novel peptidomimetic small molecule that induces tumor-selective apoptosis and modulates the WNT/β-catenin pathway via binding with the mitosis protein 68K (Sam68). Preclinical data showed its selective inhibitory activity against the Wnt gene reporter and low expression of the target genes β-catenin, cyclin D1 and survivin. CWP232291 is being tested in patients with acute myeloid leukemia (NCT01398462) and relapsed or refractory myeloma (NCT02426723) [6].
Genistein, a dietary compound found in soy-based foods, is thought to mediate antitumor activity through pleiotropic mechanisms that may include inhibition of the Wnt pathway [2]. The results of the phase I and II studies devoted to the assessment of the clinical efficacy of genistein and other isoflavone compounds from soybeans in cancer treatment and chemoprevention did not confirm those assumptions.
OMP-54F28 (Ipafricept) is a fusion protein that merges the Fc immunoglobulin domain with the cysteine-rich domain of the frizzled family receptor 8 (Fzd8), which competes with the native Fzd8 receptor for its ligands and counteracts Wnt signaling. Phase I dose escalation has shown that OMP-54F28 is well tolerated by patients, and dose escalation group studies with standard therapy are currently underway for advanced hepatocellular carcinoma (NCT02069145), pancreatic cancer (NCT02050178), and recurrent platinum-sensitive ovarian cancer (NCT02092363) [7]. OMP-18R5 is another monoclonal antibody that targets curled receptors. Currently, it is in phase 1 clinical trials administered in combination with paclitaxel to patients with metastatic breast cancer. DKN-01 is a humanized monoclonal antibody with neutralizing activity against Dickkopf-1 (Dkk-1). The basis for conducting clinical trials using these agents in combination with chemotherapeutic agents is the compelling preclinical evidence of their synergistic activity.
A few more strategies against Wnt/β-catenin-mediated carcinogenesis have been assessed in a number of preclinical studies, though they are yet to undergo clinical trials. One promising approach involves inducing or stabilizing the β-catenin 'destruction complex,' thereby diminishing its intracellular levels and inhibiting its transcriptional activity. Axin, a key component of the β-catenin degradation complex, is limited in concentration, and its stability is regulated by tankyrase. Tankyrase is an essential enzyme responsible for the poly(ADP-ribosylation) of axin, leading to its proteasomal degradation [9]. Waler et al. demonstrated the antitumor effects of tankyrase inhibition in colorectal adenoma and adenocarcinoma models. However, potential side effects such as diarrhea and intestinal toxicity were found to be associated with this therapy. Inhibition of tankyrase has also been shown to counteract resistance to PI3K and AKT inhibitors in sphere cultures derived from colorectal cancer patients and mouse tumor xenografts. A high level of nuclear β-catenin typically indicates resistance to PI3K and AKT inhibitors. Treatment combining a WNT/tankyrase inhibitor effectively reduced nuclear β-catenin levels, overcame resistance to PI3K and AKT inhibitors, and inhibited tumor growth.
Another promising strategy to reduce signaling along the canonical Wnt/β- catenin pathway is to antagonize β-catenin/TCF mediated transcription. Since the regulation of the β-catenin/TCF complex transcription requires several co-activators, especially cAMP-response element binding protein (CREB)-binding protein (CBP), this protein complex is being considered a potential target for Wnt-pathway inhibition. INT-001, a specific CBP antagonist, exhibited antitumor activity in preclinical models of tamoxifen-resistant pancreatic, colon, and breast cancer [17].
Numerous retrospective studies and randomized controlled clinical trials have demonstrated the chemopreventive role of aspirin, especially in the development of colorectal neoplasia. Given the critical importance of Wnt dysregulation in colorectal carcinogenesis, the interaction between aspirin and canonical Wnt signaling has become the subject of research [7]. By inhibiting cyclooxygenase-2 (COX-2), aspirin decreases prostaglandin E2 (PGE2) release, thereby reducing Wnt activity. Interestingly, the effect of aspirin on cancer cells in vitro led to the inhibition of the Wnt pathway due to increased β-catenin ubiquitination and subsequent degradation, independent of the COX-2 inhibition [9].
Conclusions
The Wnt/β-catenin pathway is increasingly acknowledged as a vital target for anticancer therapy, with several inhibitors currently at different stages of clinical development. The expanding role of immunotherapy in oncology, coupled with recent insights into the Wnt pathway's involvement in cancer-related immune regulation, could add a new dimension to this field of therapeutic development. To date, research has predominantly focused on tumors with upregulated Wnt/β-catenin signaling, such as colorectal cancer. However, given their significant role in immunomodulation, Wnt inhibitors might have broader applications in cancers like melanoma, lung, and kidney cancer, where immunotherapy has gained prominence. It is crucial to understand the precise role of Wnt signaling in immunomodulation more thoroughly before its integration into clinical practice.
References
[1] Cai J, Maitra A, Anders RA, Taketo MM, Pan D. Beta-catenin destruction complex - independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Gene Dev. 2015;29:1493-506.
[2] Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D, Rimm DL, McMahon M, Bosenberg M. Beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanoma. Cancer cell. 2011; 20:741-54.
[3] Konsavage Jr WM, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730-9.
[4] Merino VF, Cho S, Liang X, Park S, Jin K, Chen Q, Pan D, Zahnow CA, Rein AR, Sukumar S. STAT3, beta-catenin, and IGF-1R inhibitors sensitize murine PIK3CA mutant breast cancer to PI3K inhibitors. Mol Oncol. 2017;11(5):552-66.
[5] Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. New nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231.
[6] Riese J, Yu X, Munnerlyn A, Eresh S, Hsu SC, Grosschedl R, Bienz M. LEF-1, nuclear factor coordinating signaling from wingless and decapentaplegic. Cell. 1997;88:777-87.
[7] Roelink H, Wagenaar E, Lopes da Silva S, Nusse R. Wnt-3, a gene activated by provirus injection into mouse mammary tumors, is homologous to int-1/Wnt-1 and is commonly expressed in mouse embryos and adult human brain . Proc Natl Academy Sci U S A. 1990;87:4519-23.
[8] Ayadi M, Bouygues A, Ouare D, Ferrand N, Shuaib S, Thierry J.P., Muchardt S, Sabbah M, Larsen AK. Chronic chemotherapy stress promotes stem cell development and WNT/beta- catenin signaling in colorectal cancer cells: implications for the clinical use of inhibitors of WNT signaling. oncotarget. 2015;6:18518-33.
[9] Behrens J., Dzherkhov B.A., Wurtele M., Grimm J., Asbrand S., Wirtz R., Kuhl M., Wedlich D., Birchmeier V. Functional interaction of the axin homologue, conductin, with beta- catenin , APC and GSK3beta. The science. 1998;280: 596-9.
[10] Behrens J, von Kris JP, Kuhl M, Brun L, Wedlich D, Grossheadl R, Birchmeier W. Functional interaction of beta-catenin with transcription factor LEF-1. Nature. 1996; 382:638-42.
[11] Burton A, Sahut-Barnola I, Lambert-Lenglet C, de Jussino C, Damon-Subeyran C, Louise E, Taketo Mm, Tissier F, Bertera J., Lefrancois-Martinez A.M. and others. Constitutive activation of beta-catenin causes adrenal hyperplasia and promotes the development of adrenal cancer. Hum Mol Genet. 2010;19:1561-76.
[12] Boulter L., Guest R.W., Kendall T.J., Wilson D.H., Wojtacha D., Robson A.J., Ridgway R.A., Samuel K., Van Rooyen N., Barry ST. and others. WNT signaling stimulates the growth of cholangiocarcinoma and can be pharmacologically suppressed. J Clin Invest. 2015;125:1269-85.
[13] Brunner E, Peter O, Schweiser L, Basler K. Pangolin encodes a Lef-1 homologue that acts downstream of the armadillo to transmit the wingless signal in Drosophila. Nature. 1997;385:829–33.
[14] Wang V, Zhong V, Yuan Jay, Yan S, Hu S, Tong Wu, Mao Wu, Hu T, Zhang B, Song G: Involvement of WNT/beta-catenin signaling in mesenchymal stem cells promotes metastatic growth and chemoresistance of cholangiocarcinoma . Oncotarget 2015.
[15] van Ooyen A, Qui V, Nusse R. Nucleotide sequence of the human mammary int-1 oncogene; evolutionary conservation of coding and non-coding sequences. Embo J. 1985; 4:2905-9.
[16] Veeman M.T., Axelrod J.D., Moon R.T. Second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. dev cell. 2003;5:367-77.
[17] Vermeulen L., De Souza E.F., van der Heyden M., Cameron K., de Jong J.H., Borowski T., Tuynman J.B., Todaro M., Merz S., Rodermond H. et al. Wnt activity determines colon cancer stem cells and is regulated by the microenvironment. Natural Cell Biol. 2010;12:468– 76.
[18] Gallahan D, Callahan R. The mouse mammary tumor associated INT3 gene is a unique member of the NOTCH (NOTCH4) gene family. Oncogene. 1997;14:1883–90.
[19] Gallahan D, Callahan R. Mammary tumorigenesis in wild mice: identification of a novel int locus in mammary tumors induced by mouse mammary tumor virus (Czech II). J Virol. 1987;61:66-74.
[20] Gallagher CJ, Rambow F, Kumasaka M, Champeval D, Bellakosa A, Delmas W, Larue L. Beta-catenin inhibits melanocyte migration but causes melanoma metastasis. Oncogene. 2013;32:2230–8.
[21] Gojou S, Grabar S, Fassnacht M, Ragazon B, Lone P, Liebe R, Shokri I, Odeburg A, Royer B, Sbiera S, et al. Beta-catenin activation is associated with specific clinical and pathological characteristics and poor outcome in adrenocortical carcinoma. Clinic Cancer Res. 2011;17:328-36.
[22] Dixon S, Peters G. Potential oncogenic product associated with growth factors. Nature. 1987;326:833.
[23] Dixon S, Smith R, Brooks S, Peters G. Oncogenesis by mouse mammary tumor virus: proviral cellular gene activation in the common int-2 integration region. Cell. 1984;37:529-36.
[24] Clements V.M., Van J., Sarnaik A., Kim O.J., McDonald J., Fenoglio-Preizer S., Groden J., Lowy A.M. Beta-catenin mutation is a common cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503-6
[25] Crago A.M., Khmeletsky J., Rosenberg M., O'Connor R., Byrne S., Wilder F.G., Thorn K., Agius P., Cooke D., Sochchi N.D. et al. Near-Universal Detection of Alterations in CTNNB1 and Wnt Pathway Regulators in Desmoid-Type Fibromatosis Using Whole-Exome Sequencing and Genomic Analysis. Cancer genes of chromosomes. 2015;54:606-15.
[26] Laurent-Puig P, Legua P, Bluto O, Belgiti J, Franco D, Bino F, Monges G, Thomas G, Biulak-Sage P, Zucman-Rossi J. Genetic Changes, associated with hepatocellular carcinomas define different pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–73.
[27] Miller RK, Hong Ji, Munoz VA, McCree, police. Beta-catenin versus other armadillo catenins: an evaluation of our current view of canonical Wnt signaling. Prog Mol Biol Transl Sci. 2013;116:387-407.
[28] Molenaar M, van de Wetering M, Osterwegel M, Peterson-Maduro J, Godsave S, Korinek W, Rousse J, Destri O, Clevers H. Transcription factor XTcf-3 mediates beta-catenin- induced axis formation in Xenopus embryos. . Cell. 1996;86:391-9.
[29] Monga S.P., Mars V.M., Pediaditakis P., Bell A., Mule K., Bowen V.K., Van H, Zarnegar R., Michalopoulos G.K. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after dissociation of Met-beta-catenin in hepatocytes. Cancer Res. 2002;62:2064-71.
[30] Maureen PJ. Beta-catenin signaling and cancer. Bioassays. 1999;21:1021-30.
[31] Niers S. The complex world of WNT receptor signaling. Nat Rev Mol Cell Biol. 2012; 13:767-79.
[32] Nusse R, van Ooyen A, Cox D, Fung YC, Varmus H. A method for proviral activation of putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984; 307:131-6.
[33] Nusse R., Varmus ON. Many mouse mammary tumor virus-induced tumors contain a provirus integrated into the same region of the host genome. Cell. 1982;31:99-109.
[34] Ohalvo L.S., Whittaker K.A., Condelis J.S., Pollard J.W. Analysis of the expression of macrophage genes that promote tumor invasion confirms the role of Wnt signaling in mediating their activity in primary breast tumors. J Immunol. 2010;184:702-12.
[35] Fagotto F. Looking beyond the Wnt pathway to the deep nature of beta-catenin. Representative EMBO 2013;14:422-33.
[36] Huber O, Korn R, McLaughlin J, Osugi M, Hermann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mechdev. 1996;59:3-10.
[37] Chang GB, Kim Ji, Cho SD, Park KS, Chung Ji, Lee HI, Hong YS, Nam Jay S. Blockade of Wnt/beta-catenin signaling inhibits breast cancer metastasis by inhibiting a CSC- like phenotype. sci rep. 2015;5:12465.
[38] Chiang J.M., Chow Yu.H., Chen T.K., Ng K.F., Lin J.L. Expression of nuclear beta- catenin is closely associated with ulcerative growth of colorectal carcinoma. Br J Cancer. 2002;86:1124–9.
[39] Shen T, Zhang K, Seagal GP, Wei S.: Predictive value of E-cadherin and beta-catenin in triple-negative breast cancer. Am J Clin Pathol 2016.